Resonance Structure No3
Fundamental Concepts in Organic Reaction Mechanism. So each oxygen needs its turn at being doubly bonded.

Resonance Structures For No3 Nitrate Ion Youtube
Resonance Structures of NO3- Nitrate ion 231243 views Apr 26 2013 There are three resonance structures for nitrate NO3-.

. It can also be represented by the. Put two electrons between atoms to. Nitrate ion polarity explained.
This resonance hybrid is illustrated below. Resonance Structures of NO 3 Ion. Resonance structures can be written for.
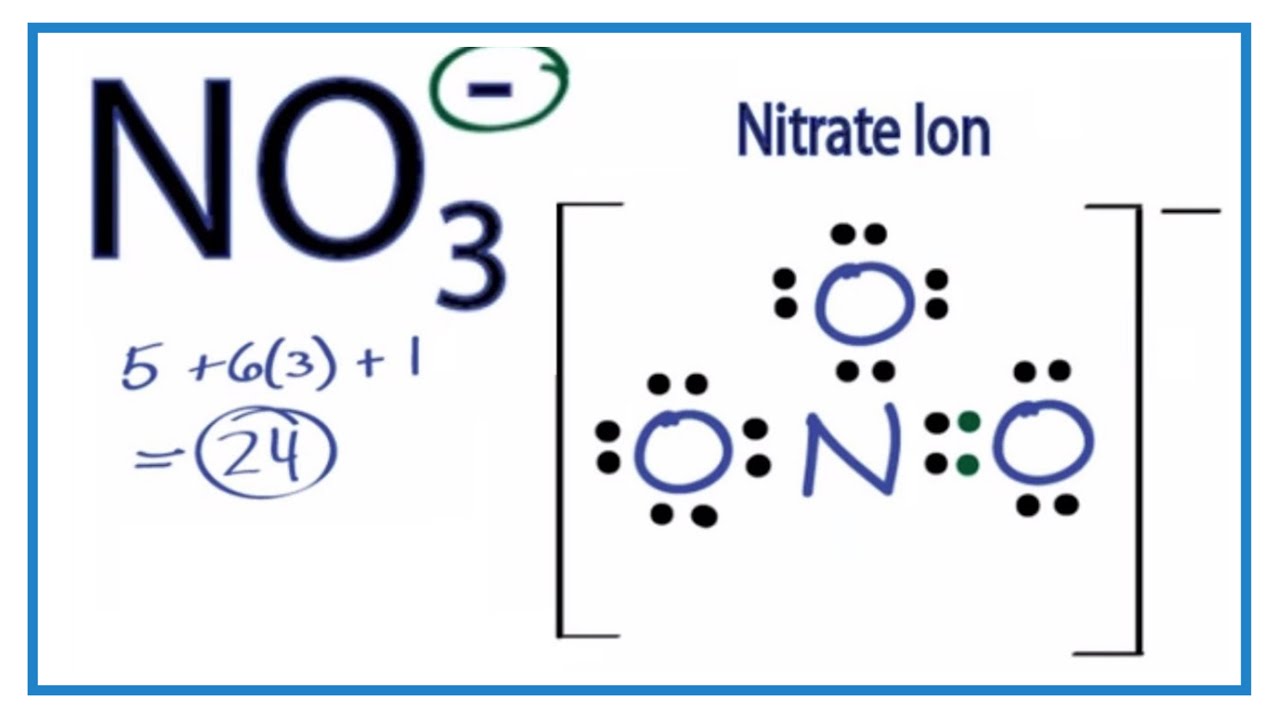
In oxygen atoms of NO3- there are lone pairs with -1 charges. Each of the three resonance structures of no3- has how many lone pairs of electrons1 05 نقطة a 7 o b 8 d 10 o e 13 o what is the formal charge on the singly bonded oxygens in the lewis structure for the carbonate 2 ion 05 نقطة a 2 o b-1 o c o d 1 e 2 o what is the formal charge on sulfur in the most favorable lewis. Draw the resonating structures of N O 3 N O 3.
You can decide this from looking about whether atoms have lone pairs or double bonds. The nitrate ion contains three N-O single bonds. How many resonance structures are there for NO3-.
With that total electrons around nitrogen atom is going to be ten. These salts exist abundantly in nature and find themselves used in a variety of applications. For the lewis structure of NO3- N is the central atom and there are 3 oxygens surrounding it.
It is widely used for manufacture of fertilizers nutrients and many explosives. Find the total valence electrons for the NO3 - molecule. CHEMISTRY 101 - Drawing Lewis Structures.
The nitrate ion contains one N-O single bond and two NO double bonds. It is a polyatomic ion with chemical formula NO3. Resonance Structures Basic Introduction - How To Draw The Resonance Hybrid Chemistry.
The formula of NO3 formal charge calculation is Total number of valance electrons number of electrons remain as nonbonded number of electrons involved in bond formation2. If a resonance hybrid of this polyatomic ion is drawn from the set of Lewis structures provided above the partial charge on each oxygen atom will be equal to - ⅔. It is derived from Nitric acid HNO3.
Asked Nov 27 2020 in Chemistry by Panna01 473k points closed Nov 27 2020 by Panna01. Hydrogen H always goes outside. The net charge on the central atom remains 1.
Resonance structures can be written for. Organic Chemistry - Some Basic Principles and Techniques. A diet rich in Nitrate elevates endurance and increases.
Check the number of electrons by simply. View resonance structuresdocx from BIOLOGY 171 at Dekalb High School. Resonance structures should have the same number of electrons do not add or subtract any electrons.
Let us calculate the formal charge of NO 3. The oxygen atoms which are singly bonded to the nitrogen hold a charge of. We can draw three resonance structures for NO3-.
Resonance Structures of NO3-1 nitrate ion. A step-by-step explanation of how to draw the NO3- Lewis Dot Structure Nitrate ionFor the NO3- structure use the periodic table to find the total number o. One of the oxygens has a double bond with N and the other two oxygens have a single bond with N because nitrogen is in period 2 so it cannot have more than 4 bonds.
The chemical formula NO3 represents the Nitrate ion. Put the least electronegative atom in the center. Describe the resonance hybrid of the nitrate ion.
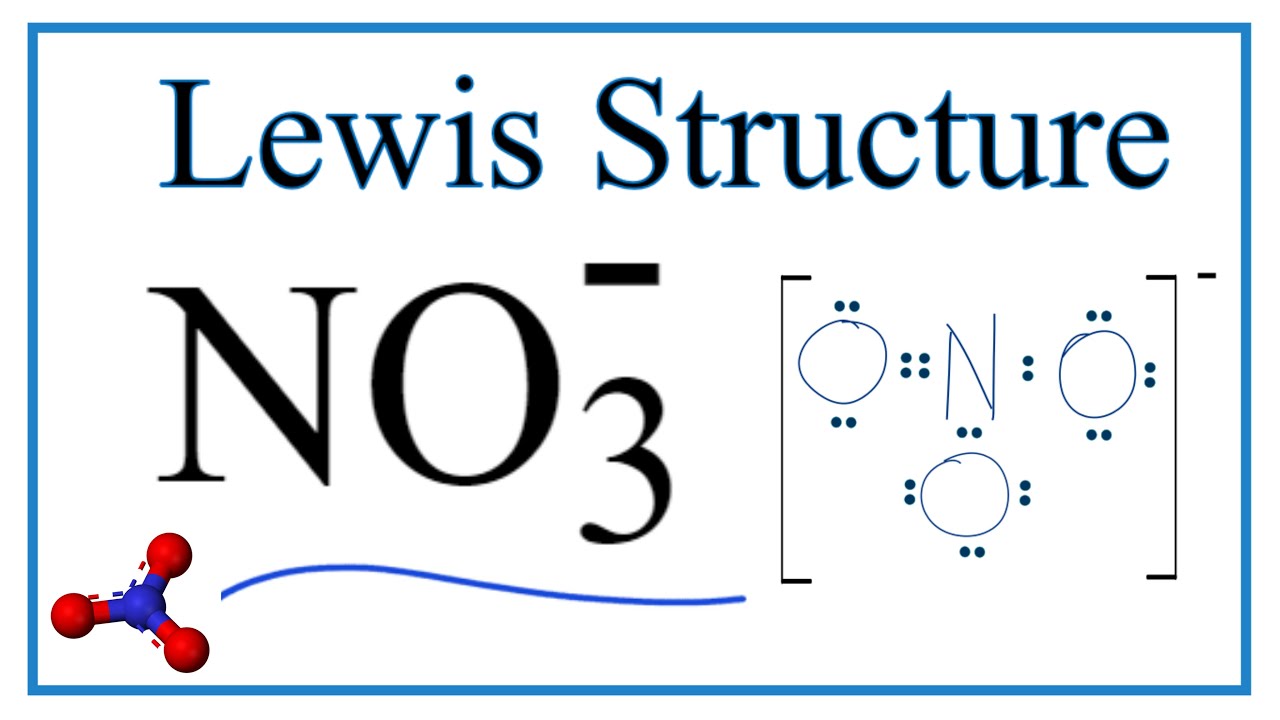
Draw the Lewis structure for NO 3- including any valid resonance structures. The third oxygen is bonded to nitrogen by a double bond. All the bond lengths are the same because the molecule has 3 resonance structures.
The total valence electron is 24 for drawing NO3 Lewis structure and it show molecular geometry is trigonal planar. Resonance hybrid structure helps to calculate the formal charge of each atom in a molecule. Thus there are three resonance forms for nitrate anion.
The three possible resonance structures of NO 3 are illustrated below. It is going to break octal rule because nitrogen atom cannot keep more than eight electrons in its last shell. Each resonance form shows two oxygens with a negative charge while the nitrogen is positively charged.
Salts containing the Nitrate ion are referred to as Nitrates. Steps to draw resonance structures for NO 3- You can convert a lone pair of one oxygen atom which already has three lone pairs to make a bond with nitrogen atom. It is singly bonded to 2 oxygen atoms and doubly bonded to 1 oxygen atom.
It depends which oxygen you choose to be double more more. But actually the NO3-1 ion is not adequately represented by this one Lewis structure. In NO3 molecule central nitrogen atom has no lone pair three oxygen atoms has each of.
Nitrogen is considered to be the central atom in the nitrate ion.
No3 Lewis Structure How To Draw The Lewis Structure For No3 Youtube

No3 Resonance Structures Nitrite Ion

Lewis Structure How Is The Nitrate Ion No3 Formed Chemistry Stack Exchange
Resonance Structures For No3 Nitrate Ion Youtube
0 Response to "Resonance Structure No3"
Post a Comment